Present in United Kingdom
Notifiable – see ‘Report a sighting’ below
Scientific name – Phytophthora kernoviae (P. kernoviae)

Phytophthora kernoviae (P. kernoviae) is a water mould organism which causes disease on the aerial (above-ground) parts of a wide range of species of trees, shrubs and other plants, including forest and woodland species. Infection can lead to death of the host plant in some cases, and it is highly destructive on some species.
It was initially spelled P. kernovii: see ‘Origins and background’ below for further details.
P. kernoviae has been recorded in Great Britain, the Republic of Ireland, Chile and New Zealand.
It has been found in all three countries of Great Britain, with the highest concentration of confirmed cases in the counties of Devon and Cornwall in South-West England.
It has not been recorded in Northern Ireland.
The principal hosts of P. kernoviae in UK woodland and heathland are rhododendron, especially Rhododendron ponticum, and bilberry (Vaccinium myrtillus), also known as blaeberry and winberry. Hundreds of rhododendron plants in woodlands have been heavily diseased or killed by it, as have areas of bilberry in south-west England and Scotland
Outside woodland, P. kernoviae can infect a wide range of other ornamental plants, especially magnolia, camellia and pieris species. It has had a significant impact in parks and gardens, particularly at certain specific locations in South-West England.
Other trees and woodland plants known to be susceptible to P. kernoviae include:
European beech (Fagus sylvatica), Chilean hazelnut (Gevuina avellana), tulip tree (Liriodendron tulipifera), winter’s bark (Drimys winterii), Holm oak (Quercus ilex), sweet michelia (Michelia doltsopa), cherry laurel (Prunus laurocerasus), ivy (Hedera helix), variegated holly (Ilex aquifolium), willow-leaf podocarp (Podocarpus salignus), radiata or Monterey pine (Pinus radiata), magnolia species, and ‘English’ or pedunculate oak (Quercus robur).
Among our native trees, hundreds of European beech trees have been infected in South-West England, as have a very small number of pedunculate oak trees.
Infected winter’s bark trees produce particularly large quantities of the spores which spread the disease.
A full list, along with the symptoms it causes on each species, is available on this factsheet.
From a woodland and natural environment point of view, our main current concerns are for beech trees, bilberry and related ecosystems.
P. kernoviae is capable of causing the same degree of damage to beech trees as that caused by its very destructive relative, Phytophthora ramorum. Our research suggests that only beech (and oak) trees standing within about 5 metres of heavily infected plants such as rhododendron are at risk from infection. It also suggests that infected trees are not contagious and can even recover from infection. However, the long-term impact that it could have will also depend on factors such as how widely P. kernoviae is distributed and whether it mutates or hybridises to become more virulent.
Infected bilberry plants usually die. Left unmanaged, P. kernoviae could pose a significant threat to bilberry and its important role in the ecology of heathland and some types of woodland, grassland and peat bog. However, P. kernoviae infection has proven difficult to manage in woodland bilberry – it appears to be able to cycle between generations of dying and emerging bilberry plants, and intervention can have a negative subsequent impact on the woodland vegetation mix.
Bilberry is also closely related to blueberry (V. corymbosum), so there could also be significant economic implications for the blueberry-growing industry were it to infect blueberries. Blueberries and other susceptible species in trade are monitored for infection by the Animal & Plant Health Agency (APHA).
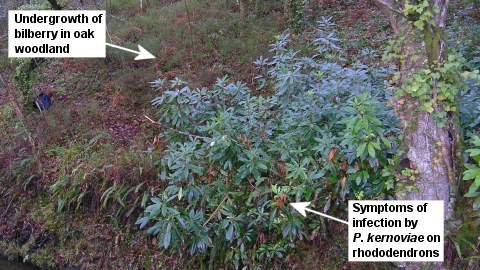
The highly susceptible Rhododendron ponticum is an invasive, non-native presence in many British woodlands. The ability of infected R. ponticum shrubs to produce large quantities of P. kernoviae spores magnifies the threat to our native plants in woodland and other habitats. The picture above shows an infected rhododendron plant standing next to bilberry plants and oak trees in woodland.
P. kernoviae can also infect a wide range of ornamental garden species. This means there could be significant economic implications for the nursery and garden centre industries, and heritage gardens, if the disease is not managed to minimise its spread and impact. However, there were no findings in trade in the three years to 2019.
In summary, P. kernoviae infection causes the following symptoms.



Pictured above are bilberry plants at an early stage of P. kernoviae dieback.
When P. kernoviae invades the bark of European beech it kills extensive areas of inner bark, often extending 10 metres (30 feet) or more up the stem. This necrotic zone oozes a black, sticky fluid, forming ‘bleeding cankers’ (top picture).
The few pedunculate oak trees which have been infected by neighbouring rhododendron bushes have also developed bleeding cankers. However, our monitoring of infected trees suggests that the bleeding cankers are not contagious, and the trees might recover from infection.
A wide range of magnolia species and magnolia hybrids have been infected by P. kernoviae, usually picking up the infection from nearby infected rhododendron plants. Our research shows that P. kernoviae can persist on infected magnolia tissues over winter, and then re-infect the new flowers and leaves which form the following spring. The most common symptom in magnolia is leaf spot (pictured below), although some species also show bud blast (premature cessation of flower development) and blossom blight.

Further help with identifying the disease is available from this factsheet.
P. kernoviae infection is difficult to diagnose without laboratory testing, because many of the symptoms are similar to those caused by other pathogens (disease-causing organisms). Owners of the susceptible plant species exhibiting these symptoms may request a diagnosis from our Tree Health Diagnostic & Advisory Service. We might ask for leaf or other tissue samples so that we can screen for P. kernoviae, as well as P. ramorum and other pathogens. Please note that there might be a fee for this service.
We particularly encourage tree and plant professionals to be vigilant and report suspected sightings of P. kernoviae infection.
Please note that TreeAlert and TreeCheck both require photographs to be uploaded. These should be clear, well-lit, close-up pictures of symptoms.
Alternatively, suspected sightings can be made directly to the relevant plant health authority. This is the preferred route for suspected sightings made on trade premises, such as nurseries and garden centres, and for suspected sightings on non-forest plants.
In all cases, provide precise details of the location and, if possible, clear, well lit, close-up photographs of the symptoms.
There is no cure for P. kernoviae infection, so the only effective treatment is to remove and destroy infected plants, preferably before their next sporulation season (the period when they produce the spores which spread the disease). It can be helpful to draw up a management plan which identifies the risks, actions and mitigations to be put in place in parallel with any Statutory Plant Health Notice (SPHN) issued. (SPHNs are issued by plant health authorities requiring owners to take action to mitigate the risk from a plant pest or disease.)
We recommend that owners and managers of P. kernoviae-infected plants follow the management guidance for Phytophthora ramorum infection.
We have developed the following guidance for managers of affected sites.
Finally, people who work at infected sites must follow good biosecurity practice, such as cleaning and disinfecting their tools, equipment, clothes, footwear, and vehicles between sites, to prevent the spread of the pathogen by these means. Guidance on this is available on the UK Government website at gov.uk/guidance/prevent-the-introduction-and-spread-of-tree-pests-and-diseases.
A Statutory Instrument came into force in December 2004 to help contain the disease, and a control area was scheduled in the Plant Health (Phytophthora Kernovii Management Zone) (England) Order 2004. The purpose of the order, which was revoked in 2014, was to supplement the powers available under general plant health legislation, and to enable inspectors to close footpaths for the purpose of carrying out eradication action. The order also prohibited the removal of all host plants from the zone without permission.
Our research (below) helped us understand the process of infection and means of spread. Some of the known infected sites in South-West England were designated as experimental sites so that we could learn more about its impact and management in different environments.
Initially we knew very little about the organism, so as a precautionary measure to prevent or limit its spread the plant health authorities had all infected plants destroyed in the same way as for P. ramorum. However, our monitoring evidence suggests that trees which become infected do not themselves become a source of infection, and might even recover.
Grants provided until 2014 for removing R. ponticum from sites infected with P. ramorum and P. kernoviae helped to reduce the organisms’ impact by destroying plants which might otherwise have spread the diseases which they cause. In addition, regular inspections by the plant health authorities for infected plants on trade premises, such as nurseries and garden centres, have helped to prevent the organism from being introduced from trade premises to new sites.
Information about P. kernoviae was given to the European Union (EU) Plant Health Committee, and all EU Member States were notified that there was a new phytophthora pathogen affecting beech and rhododendron.
We also supplied descriptions in the form of morphological and molecular profiles to enable scientists world-wide to identify it or rule it out. EU Member States now routinely look for P. kernoviae as part of obligatory surveys for P. ramorum.
Our scientists first isolated, identified and characterised P. kernoviae as a new species in 2003. Our research, funded by Defra and the Forestry Commission’s Phytophthora Programme, investigated the process of infection and how the organism spreads. This enabled our scientists to formulate appropriate management strategies and advice for owners and managers of affected sites.
They have also taken part in a research project to investigate whether P. kernoviae could pose a threat to North American forest plants. This was in part motivated by the observation that P. kernoviae and P. ramorum have an overlapping geographic range in Great Britain, and often concurrently invade the same site and infect some of the same plant species. Different genetic forms of the P. ramorum organism are the cause of sudden oak death in oak trees (Quercus spp.) and tanoak trees (Lithocarpus densiflorus) in the USA, so it was suggested that P. kernoviae might also invade North American forests threatened by P. ramorum.
Our related work has covered:
We have produced the following materials based on our research.
Other research-related material is listed below under ‘Related materials’.
P. kernoviae was first found in October 2003 when, during surveys for P. ramorum, Forest Research scientists isolated and characterised a previously unknown phytophthora in a large bleeding canker on a mature beech tree in Cornwall.
About the same time scientists from the Central Science Laboratory (now part of Fera Science) isolated an identical new organism from established rhododendrons, also from South-West England. It was confirmed as being the same organism.
The scientist who discovered it on a beech tree was Professor Clive Brasier, Emeritus Mycologist at Forest Research. He named it Phytophthora kernovii after Kernow, the Cornish name for Cornwall, where it was first identified. The spelling was later changed to kernoviae.
Since then it has also been found elsewhere in Great Britain, mainly in South-West England, but occasionally at other locations in England as well as Scotland and Wales. Most sites have been in western regions, where the wetter climate suits water moulds. All submitted samples from known host plants are now routinely analysed for P. ramorum and P. kernoviae.
The pathogen grows best at a temperature of about 18 °C, with an upper limit of 26 °C, which suggests it is adapted to a temperate climate.
There is evidence that P. kernoviae might have been present in New Zealand for more than 50 years. There, it has been found in soil under radiata pine trees in Trounson Kauri Park in northern New Zealand as well as infecting the needles of radiata pine, and on a custard apple tree (Annona cherimola) in an abandoned orchard. Custard apple is a native species of South America.
In Chile it has been found on Chilean hazelnut, and on winter’s bark trees in native forests.
Its presence in Chile and New Zealand, and findings of the disease in Southern Hemisphere species such as Podocarpus, Drimys and Gevuina plants in Britain, all point to a possible origin there. However, origins in the temperate forests of the eastern Himalayas, China or Taiwan have also been suggested.